ORDER NEW LITERATURE REVIEW ON POLYSTYRENE ASSIGNMENT AT NOMINAL PRICE!
ADM80016 Masters Project Assignment - Swinburne University of Technology, Australia
Project - Weight capacity of Laminated Polystyrene used in campervans
In this project, evaluate the weight capacity at atleast 3 different locations on the provided sample. A brief literature survey to compare the evaluated properties with published values will also need to be performed.
Answer - LITERATURE REVIEW ON POLYSTYRENE USED IN CAMPERVANS
LITERATURE REVIEW
2.1 Overview
The literature review provides an understanding and general theoretical background on the weight capacity of Laminated Polystyrene used in campervans by various authors and research scholars. The review gives a brief insight into polystyrene, the application of laminated polystyrene and an insight into the employment of doors made of polystyrene in campervans.
2.1 Polystyrene
In this report, Jeffery et.al brings out the various characteristics of polystyrene. The report gives a brief description of the use of polystyrene to produce a polymer which has reduced flammability characteristics. The combination of nano-composites like montmorillonite and fluorohectorite along with polystyrene produced polypropylene-graft-maleic anhydride and polystyrene layered silicates. Under controlled conditions, these properties were tested with the help of a continuous beam of diffractive rays and a high intensive grain microscope was used to study the combustion residues [1].
Polystyrene-co-acrylonitrile is the most productive of all the polymers. It is known for its tensile strength, ability to withstand loads and has excellent properties related to mechanical. As far as its ability to react with chemical is concerned it is quite receptive. The final products produced are of yellowish in color as suggested by Cabaleiro-Lago in his paper. Polystyrene thereby comprises a structure that is quite suitable for suffocation.
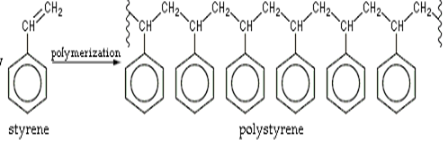
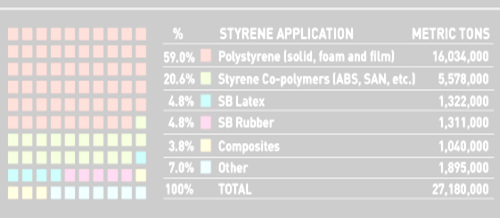
Polystyrene is a type of thermoplastic which is capable of producing products that are not completely assembled. These are amorphous in nature. The process of formation of these polymers is not that much difficult. The most probable application of these polymers is thin layered sheets, bath sponges and the thin layer of sulfonate. A major portion of the need for thermoplastic is fulfilled by polystyrene. They account for around seven to eight percent of the needs. Polystyrene does not get easily dissolved with acids or bases.
GET BENEFITTED WITH QUALITY LITERATURE REVIEW ON POLYSTYRENE ASSIGNMENT HELP SERVICE OF EXPERTSMINDS.COM!
As it is amorphous in nature it is extremely luminous. Polystyrene is a bad conductor of electricity. The manufacturing of polystyrene products is quite easy. This is because it has a glass transition temperature that is quite less. The temperature at which the polystyrene can act as neither a liquid nor as a solid upon heating. Molding the polymer becomes less difficult. Apart from these properties polystyrene attracts atoms of hydrogen and carbon. It has its own set of drawbacks. It can break easily on continuous exposure to ultraviolet radiations i.e. the sunlight. Due to exposure to the atmosphere, its ability to withstand load is reduced. The boiling point is reduced due to its amorphous nature. In spite of all these drawbacks, they are the most productive polymers to be employed.
Polystyrene has found its application in the field of construction. ASTM C578 has excellent thermal properties. This type of polystyrene is employed for thermal insulation. The combination of nano-composites along with polystyrene produced polypropylene-graft-maleic anhydride and polystyrene layered silicates. These silicates can be employed for thermal insulation. It is also a suitable material that can be employed in the packaging industry. Polystyrene is employed in pharmaceuticals in a productive and effective manner. These materials can be transported by maintaining them in thermal control conditions. The cellar structure of the polystyrene proves to be highly resistant to the flow of heat. The polystyrene's thermal conductivity varies with temperature.
Polystyrene products begin to melt when exposing to high temperatures. The process of development of Polystyrene products is using the Dow process. The boards are formed using this process. The maximum temperatures it can be maintained is 75 °C. Applications of adhesives that are highly soluble are suggested. Due to exposure to the atmosphere, its ability to withstand load is reduced. The boiling point is reduced due to its amorphous nature. In spite of all these drawbacks, they are the most productive polymers to be employed. The employment of adhesive which contains very little amount of solvent is suggested.
This type of polystyrene is employed for thermal insulation. They are highly compactable and are responsible for the production of doors. On direct contact, the molecules begin to melt and as a result, the door starts to deform. The composition of the door accounts for around 65% of polystyrene while the remaining percent accounts for aluminum. Several investigations have been carried based on these combinations and enhancements in properties have been seen in most cases. The properties of polystyrene were clearly studied before employing this design to the campervans. The manufacturing of polystyrene products is quite easy. This is because it has a glass transition temperature that is quite less. The temperature at which the polystyrene can act as neither a liquid nor as a solid upon heating.
Thomas and Flory in their report gave the experimental results according to which the molecular weight of polystyrene depends on the coefficient of viscosity, coefficient of temperature, bulk viscosity, glass temperature, and specific volume. Polystyrene-co-acrylonitrile is the most productive of all the polymers. It is known for its tensile strength, ability to withstand loads and has excellent properties related to mechanical. As far as its ability to react with chemical is concerned it is quite receptive. The final products produced are of yellowish in color.
ORDER NEW COPY OF LITERATURE REVIEW ON POLYSTYRENE ASSIGNMENT & GET HIGH QUALITY SOLUTIONS FROM SUBJECT'S TUTORS!
As the density increases the strength of the polystyrene molecule increases. The packaging industry entirely depends on the performance of polystyrene. It provides a cushioning effect and that is the main feature for it being preferred in packaging. It provides a cushioning effect to seats as well. In such type of seats, the cushioning effect is for the absorption of shock. Polystyrene changes in between temperatures 170C and 430C.
The main drawback of polystyrene is that it cannot be disposed of easily. It is a non-biodegradable form of waste. It is extremely repulsive to sunlight. Polystyrene has been a major source of waste accumulation in the ocean. Though it is being considered as one of the most productive forms of plastics it is being replaced by other composite thermoplastics because of the menace it can create. Expandable polystyrene is hard to dispose of as they occupy a large amount of space. Even after recycling polystyrene it is not suitable to employ it over a period of time.
2.2 Application of polystyrene
Clark et.al in his report worked on Blocks of copolymers and calculated the angular measurements. The ESCA determined the morphology of several structures of such blocks The poly-dimethylsiloxane and polystyrene were briefly examined and the angle of contact was studied. Poly-dimethylsiloxane makes up for the main component. The elastic properties of the component were studied. By the process of photoionization, the elastic properties were compared between poly-dimethylsiloxane and polystyrene. The thickness of the outer layer calculated by EPA is determined to be ∼13 and 40 Å. Polystyrene has found its application in the field of construction. ASTM C578 has excellent thermal properties. This type of polystyrene is employed for thermal insulation.
The combination of nano-composites along with polystyrene produced polypropylene-graft-maleic anhydride and polystyrene layered silicates. These silicates can be employed for thermal insulation. It is also a suitable material that can be employed in the packaging industry. Polystyrene is employed in pharmaceuticals in a productive and effective manner. These materials can be transported by maintaining them in thermal control conditions. The cellar structure of the polystyrene proves to be highly resistant to the flow of heat. The polystyrene's thermal conductivity varies with temperature [5]. Apart from these properties polystyrene attracts atoms of hydrogen and carbon. It has its own set of drawbacks. It can break easily on continuous exposure to ultraviolet radiations i.e. the sunlight. Due to exposure to the atmosphere, its ability to withstand load is reduced. The boiling point is reduced due to its amorphous nature. In spite of all these drawbacks, they are the most productive polymers to be employed.
The packaging industry entirely depends on the performance of polystyrene. These materials can be transported by maintaining them in thermal control conditions. The cellar structure of the polystyrene proves to be highly resistant to the flow of heat. It provides a cushioning effect and that is the main feature for it being preferred in packaging. It provides a cushioning effect to seats as well. In such type of seats, the cushioning effect is for the absorption of shock. Polystyrene changes in between temperatures 170C and 430C.
SAVE TOP GRADE USING LITERATURE REVIEW ON POLYSTYRENE ASSIGNMENT HELP SERVICE OF EXPERTSMINDS.COM!
Shin's work comprised of separation of water-oil emulsion. It comprises of minute droplets. The droplets which are very minute having 100 μmare very difficult to separate. Secondary the process of coalescence depends on the properties of the droplets. It depends upon the size and performance of the droplets. This affects the surface area of the emulsion. These fibers are mixed with micro glass fibers to modify the glass fiber filter media. The cellar structure of the polystyrene proves to be highly resistant to the flow of heat. ASTM C578 has excellent thermal properties. This type of polystyrene is employed for thermal insulation. The combination of nano-composites along with polystyrene produced polypropylene-graft-maleic anhydride and polystyrene layered silicates. Polystyrene is employed in pharmaceuticals in a productive and effective manner. The process of formation of these polymers is not that much difficult. The most probable application of these polymers is thin layered sheets, bath sponges and the thin layer of sulfonate. A major portion of the need for thermoplastic is fulfilled by polystyrene. They account for around seven to eight percent of the needs. These materials can be transported by maintaining them in thermal control conditions.
Bertoch et.al in his report stated that polystyrene products begin to melt when exposing to high temperatures. The process of development of Polystyrene products is using the Dow process. The boards are formed using this process. The maximum temperatures it can be maintained is 75 °C. The application of adhesives that are highly soluble is suggested. The employment of adhesive which contains very little amount of solvent is suggested. This type of polystyrene is employed for thermal insulation. They are highly compactable and are responsible for the production of doors. On direct contact, the molecules begin to melt and as a result, the door starts to deform.
The boards are tested by experimenting them in prolonged exposure to sunlight. They have to be kept from flammable materials. Under controlled conditions, these properties were tested with the help of continuous beam of diffractive rays and high intensive grain microscope was used to study the combustion residues. In order to prevent it from getting exposed to sunlight, it should be maintained in vandalized rooms and maintained in it over a period of time. The intensity of the plastic sheets should be very low since they act as a covering. The enhancement of high temperatures occurs when high-intensity colors are employed. Applications of adhesives that are highly soluble are suggested. The use of solvent-free adhesive is suggested. This type of polystyrene is employed for thermal insulation.
The experimental results according to which the molecular weight of polystyrene depends on the coefficient of viscosity, coefficient of temperature, bulk viscosity, glass temperature, and specific volume are to be studied. Knowledge and understanding of the materials based on the application, physical properties, load characteristics, and other properties are significant before manufacturing a product.
DO YOU WANT TO EXCEL IN LITERATURE REVIEW ON POLYSTYRENE ASSIGNMENT - ORDER AT EXPERTSMINDS!
Nehls et.al stated that the cellar structure of the polystyrene proves to be of highly resistant to the flow of heat. It provides a cushioning effect and that is the main feature for it being preferred in packaging. It provides a cushioning effect to seats as well. Polystyrene products begin to melt when exposing to high temperatures. The process of development of Polystyrene products is using the Dow process. The boards are formed using this process. Applications of adhesives that are highly soluble are suggested. Under controlled conditions, these properties were tested with the help of scattered diffraction and the transmission electron microscope was employed to study the combustion residues.
The droplets which are very minute having 100 μmare very difficult to separate. Secondary the process of coalescence depends on the properties of the droplets. It depends upon the size and performance of the droplets. This affects the surface area of the emulsion. These fibers are mixed with micro glass fibers to modify the glass fiber filter media. The cellar structure of the polystyrene proves to be highly resistant to the flow of heat. The need to understand the significance of materials is a must in order to understand the limits and change of properties with use. Based on the solution obtained from observation, the task for the engineers is reduced since they help in producing efficient solutions. Thus through these observations, the efficiency of the insulators is increased.
The manufacturing of polystyrene products is quite easy. This is because it has a glass transition temperature that is quite less. The temperature at which the polystyrene can act as neither a liquid nor as a solid upon heating. Molding the polymer becomes less difficult. Apart from these properties polystyrene attracts atoms of hydrogen and carbon.
Baker.S and Baker.K stressed the role of polystyrene in infrared thermography. Through the comparative analysis, these properties were tested with the help of continuous beam of diffractive rays and high intensive grain microscope was used to study the combustion residues. The combination of nano-composites like montmorillonite and fluorohectorite along with polystyrene produced polypropylene-graft-maleic anhydride and polystyrene layered silicates as stated earlier. Polystyrene-co-acrylonitrile is the most productive of all the polymers. It is known for its tensile strength, ability to withstand loads and has excellent properties related to mechanical. As far as its ability to react with chemical is concerned it is quite receptive. The final products produced are of yellowish in color.
The production of polystyrene is not as much difficult. It is not as expensive also. It finds its application in the field of packaging, optics, in the degradation of plastics, etc. It also finds its application in the field of medicine. The packaging industry entirely depends on the performance of polystyrene. These materials can be transported by maintaining them in thermal control conditions. The cellar structure of the polystyrene proves to be highly resistant to the flow of heat.
Polystyrene is employed for manufacturing bottles, containers made of plastic and other kitchen appliances. The manufacturing of polystyrene products is quite easy. This is because it has a glass transition temperature that is quite less. The temperature at which the polystyrene can act as neither a liquid nor as a solid upon heating. Molding the polymer becomes less difficult.
DON'T MISS YOUR CHANCE TO EXCEL IN LITERATURE REVIEW ON POLYSTYRENE ASSIGNMENT! HIRE TUTOR OF EXPERTSMINDS.COM FOR PERFECTLY WRITTEN LITERATURE REVIEW ON POLYSTYRENE ASSIGNMENT SOLUTIONS!
Apart from these properties polystyrene attracts atoms of hydrogen and carbon. It has its own set of drawbacks. It can break easily on continuous exposure to ultraviolet radiations i.e. the sunlight. Due to exposure to the atmosphere, its ability to withstand load is reduced. It is used in camper vans where the composition of the door accounts for around 65% of polystyrene while the remaining percent accounts for aluminum [7]. Several investigations have been carried based on these combinations and enhancements in properties have been seen in most cases. The properties of polystyrene were clearly studied before employing this design to the campervans. The manufacturing of polystyrene products is quite easy. This is because it has a glass transition temperature that is quite less. The temperature at which the polystyrene can act as neither a liquid nor as a solid upon heating started by Chuanxi, X in his study.
Polystyrene is employed in the production of injections, utensils employed for holding medical wastes, etc. They find their application in the manufacturing of burette, pipette, heat exchangers, use and throw bottles, egg holding packets, etc. Applications of adhesives that are highly soluble are suggested. Under controlled conditions, these properties were tested with the help of scattered diffraction and the high-intensity monochromatic laser microscope was employed to study the combustion residues.
T. Zhai in his report stated that apart from various other significant properties polystyrene attracts atoms of hydrogen and carbon. It has its own set of drawbacks. It can break easily on continuous exposure to ultraviolet radiations i.e. the sunlight. Due to exposure to the atmosphere, its ability to withstand load is reduced. The boiling point is reduced due to its amorphous nature. In spite of all these drawbacks, they are the most productive polymers to be employed.
Polystyrene-co-acrylonitrile is the most productive of all the polymers. It is known for its tensile strength, ability to withstand loads and has excellent properties related to mechanical. As far as its ability to react with chemical is concerned it is quite receptive. The final products produced are of yellowish in color.
Another polymer of the same caliber is the polystyrene-co-acrylonitrile-co-butadiene. It is quite repulsive to acids and bases and does not come in contact easily with chemicals. The mechanical properties are as excellent as seen in polystyrene-co-acrylonitrile.
In order to produce products that are highly resistive to heat polystyrene, they are allowed to come in contact with other structured. To achieve an enhancement in properties it is mixed with maleic anhydride. Thereby this increases the tendency of the polymer to produce products with high transition temperatures.
WORK TOGETHER WITH EXPERTSMIND'S TUTOR TO ACHIEVE SUCCESS IN LITERATURE REVIEW ON POLYSTYRENE ASSIGNMENT!
Samuel Zalipsky investigated the properties of polystyrene in combination with polyethylene glycol. The chemical properties of polyethylene glycol were processed with polystyrene to form the following combination BocNH(CH2CH2O)nCONHCH2CO2H.They have to be kept from flammable materials. Under controlled conditions, these properties were tested with the help of continuous beam of diffractive rays and high intensive grain microscope was used to study the combustion residues. In order to prevent it from getting exposed to sunlight, it should be maintained in vandalized rooms and maintained in it over a period of time. The intensity of the plastic sheets should be very low since they act as a covering [7].
The enhancement of high temperatures occurs when high-intensity colors are employed. Applications of adhesives that are highly soluble are suggested. The use of solvent-free adhesive is suggested. This type of polystyrene is employed for thermal insulation. The polystyrene's thermal conductivity varies with temperature. Apart from these properties polystyrene attracts atoms of hydrogen and carbon. It has its own set of drawbacks. It can break easily on continuous exposure to ultraviolet radiations i.e. the sunlight. Due to exposure to the atmosphere, its ability to withstand load is reduced. The boiling point is reduced due to its amorphous nature. In spite of all these drawbacks, they are the most productive polymers to be employed.
Several investigations have been carried based on these combinations and enhancements in properties have been seen in most cases. Polystyrene has made its own mark in the production of plastic products. Polystyrene is employed in pharmaceuticals in a productive and effective manner. The process of formation of these polymers is not that much difficult. The most probable application of these polymers is thin layered sheets, bath sponges and a thin layer of sulfonate. A major portion of the need for thermoplastic is fulfilled by polystyrene. They account for around seven to eight percent of the needs. These materials can be transported by maintaining them in thermal control conditions. The main drawback of polystyrene is that it cannot be disposed of easily. It is a non-biodegradable form of waste. It is extremely repulsive to sunlight. Polystyrene has been a major source of waste accumulation in the ocean. Though it is being considered as one of the most productive forms of plastics it is being replaced by other composite thermoplastics because of the menace it can create. Expandable polystyrene is hard to dispose of as they occupy a large amount of space. Even after recycling polystyrene it is not suitable to employ it over a period of time.

Even the packaging industry that has been employing polystyrene in large numbers has started opting for replacement over the years. We expect other lower-cost and lower-density resins to gain market share in traditional large volume applications of expandable polystyrene. This was determined under controlled conditions, these properties were tested with the help of scattered diffraction and the high-intensity monochromatic laser microscope was employed to study the combustion residues.
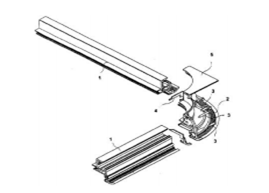
Yarey Volpe in his study stated the application of polystyrene for campervans. They are capable of withstanding loads that arise during the opening and closing of doors. These doors are provided by hinges. The composition of the door accounts for around 65% of polystyrene while the remaining percent accounts for aluminum. Several investigations have been carried based on these combinations and enhancements in properties have been seen in most cases. The properties of polystyrene were clearly studied before employing this design to the campervans. The manufacturing of polystyrene products is quite easy. This is because it has a glass transition temperature that is quite less. The temperature at which the polystyrene can act as neither a liquid nor as a solid upon heating.
ENROL WITH LITERATURE REVIEW ON POLYSTYRENE ASSIGNMENT HELP AND HOMEWORK WRITING SERVICES OF EXPERTSMINDS.COM AND GET BETTER RESULTS IN LITERATURE REVIEW ON POLYSTYRENE ASSIGNMENTS!
Molding the polymer becomes less difficult. Apart from these properties polystyrene attracts atoms of hydrogen and carbon. It has its own set of drawbacks. It can break easily on continuous exposure to ultraviolet radiations i.e. the sunlight. Due to exposure to the atmosphere, its ability to withstand load is reduced.
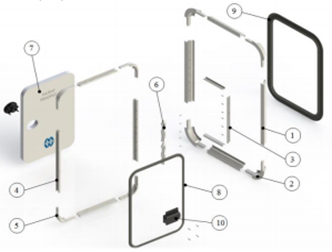
The boiling point is reduced due to its amorphous nature. In spite of all these drawbacks, they are the most productive polymers to be employed. Using CAD/CAM the mechanical constraints of using polystyrene for doors were stated.
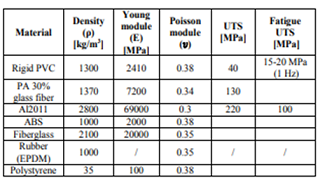
William.J.Joost in his article stated the importance of weight reduction in vehicles. There have been advancements in technology in day to day basis. Weight reduction plays a major role in deciding the orientation of the vehicle. Several materials have been employed to reduce weight in the past. Polystyrene is one of them. About six to eight percent of the fuel efficiency can be enhanced by reducing the weight of the vehicle.
In his study, Kelly. J stressed the importance of reduction in weight in vehicles. He suggested the application of composites made of aluminum and magnesium in order to reduce the weight of the vehicle. His study also comprised of employing polystyrene doors for the vehicle. This is because the manufacturing of polystyrene products is quite easy because it has a glass transition temperature that is quite less. The temperature at which the polystyrene can act as neither a liquid nor as a solid upon heating. According to Cole, molding the polymer becomes less difficult. Apart from these properties polystyrene attracts atoms of hydrogen and carbon. It has its own set of drawbacks. It can break easily on continuous exposure to ultraviolet radiations i.e. the sunlight. Due to exposure to the atmosphere, its ability to withstand load is reduced.
In his study, Doh, J. G. stated that polystyrene products are products that are not completely assembled. These are amorphous in nature. The process of formation of these polymers is not that much difficult. The most probable application of these polymers is thin layered sheets, bath sponges and the thin layer of sulfonateClark et.al in his report worked on Blocks of copolymers and calculated the angular measurements.
24/7 AVAILABILITY OF TRUSTED LITERATURE REVIEW ON POLYSTYRENE ASSIGNMENT WRITERS! ORDER ASSIGNMENTS FOR BETTER RESULTS!
The ESCA determined the morphology of several structures of such blocks The poly-dimethylsiloxane and polystyrene were briefly examined and the angle of contact was studied. Poly-dimethylsiloxane makes up for the main component. The elastic properties of the component were studied. By the process of photoionization, the elastic properties were compared between poly-dimethylsiloxane and polystyrene. The thickness of the outer layer calculated by EPA is determined to be ∼13 and 40 Å. Polystyrene has found its application in the field of construction. ASTM C578 has excellent thermal properties. This type of polystyrene is employed for thermal insulation. The major portion of the need for thermoplastic is fulfilled by polystyrene. They account for around seven to eight percent of the needs. Polystyrene does not get easily dissolved with acids or bases.
Yu, T is his paper determined that there have instances where combinations and enhancements in properties have been employed. The properties of polystyrene were clearly studied before employing this design to the campervans. The manufacturing of polystyrene products is quite easy. This is because it has a glass transition temperature that is quite less. The temperature at which the polystyrene can act as neither a liquid nor as a solid upon heating.
Fox T.G determined that polystyrene is a bad conductor of electricity. The manufacturing of polystyrene products is quite easy. This is because it has a glass transition temperature that is quite less. The temperature at which the polystyrene can act as neither a liquid nor as a solid upon heating. Molding the polymer becomes less difficult. Apart from these properties polystyrene attracts atoms of hydrogen and carbon. It has its own set of drawbacks. It can break easily on continuous exposure to ultraviolet radiations i.e. the sunlight. Due to exposure to the atmosphere, its ability to withstand load is reduced. The boiling point is reduced due to its amorphous nature. In spite of all these drawbacks, they are the most productive polymers to be employed.
The cellar structure of the polystyrene proves to be highly resistant to the flow of heat. ASTM C578 has excellent thermal properties. This type of polystyrene is employed for thermal insulation. The combination of nano-composites along with polystyrene produced polypropylene-graft-maleic anhydride and polystyrene layered silicates. Polystyrene is employed in pharmaceuticals in a productive and effective manner. The process of formation of these polymers is not that much difficult. The major portion of the need for thermoplastic is fulfilled by polystyrene. They account for around seven to eight percent of the needs. These materials can be transported by maintaining them in thermal control conditions.
The main drawback of polystyrene is that it cannot be disposed of easily. It is a non-biodegradable form of waste. It is extremely repulsive to sunlight. Polystyrene has been a major source of waste accumulation in the ocean. Though it is being considered as one of the most productive forms of plastics it is being replaced by other composite thermoplastics because of the menace it can create.
GET ASSURED A++ GRADE IN EACH LITERATURE REVIEW ON POLYSTYRENE ASSIGNMENT ORDER - ORDER FOR ORIGINALLY WRITTEN SOLUTIONS!
Expandable polystyrene is hard to dispose of as they occupy a large amount of space. Even after recycling polystyrene it is not suitable to employ it over a period of time. The combination of nano-composites along with polystyrene produced polypropylene-graft-maleic anhydride and polystyrene layered silicates. These silicates can be employed for thermal insulation. It is also a suitable material that can be employed in the packaging industry. Polystyrene is employed in pharmaceuticals in a productive and effective manner. These materials can be transported by maintaining them in thermal control conditions. The cellar structure of the polystyrene proves to be highly resistant to the flow of heat. The polystyrene's thermal conductivity varies with temperature. Apart from these properties polystyrene attracts atoms of hydrogen and carbon. It has its own set of drawbacks. It can break easily on continuous exposure to ultraviolet radiations i.e. the sunlight. Due to exposure to the atmosphere, its ability to withstand load is reduced. The boiling point is reduced due to its amorphous nature. In spite of all these drawbacks, they are the most productive polymers to be employed.
Peeling, J. suggested the application of composites made of aluminum and magnesium in order to reduce the weight of the vehicle. His study also comprised of employing polystyrene doors for the vehicle. This is because the manufacturing of polystyrene products is quite easy because it has a glass transition temperature that is quite less. The temperature at which the polystyrene can act as neither a liquid nor as a solid upon heating. According to Cole, molding the polymer becomes less difficult. Apart from these properties polystyrene attracts atoms of hydrogen and carbon. It has its own set of drawbacks. It can break easily on continuous exposure to ultraviolet radiations i.e. the sunlight. Due to exposure to the atmosphere, its ability to withstand load is reduced.
Vanderwall forces are employed to determine the properties of the material obtained between polymer chains. The attractive nature of the atoms is more since there is a large number of atoms that are so closely packed. On heating, the chain enables sliding. The weakness of the walls due to hydrocarbons provides flexibility. This is because the manufacturing of polystyrene products is quite easy because it has a glass transition temperature that is quite less. The temperature at which the polystyrene can act as neither a liquid nor as a solid upon heating.
Yang.Y suggested the application of composites made of aluminum and magnesium in order to reduce the weight of the vehicle. His study also comprised of employing polystyrene doors for the vehicle. This is because the manufacturing of polystyrene products is quite easy because it has a glass transition temperature that is quite less. The temperature at which the polystyrene can act as neither a liquid nor as a solid upon heating. According to Cole, molding the polymer becomes less difficult. Apart from these properties polystyrene attracts atoms of hydrogen and carbon. It has its own set of drawbacks. It can break easily on continuous exposure to ultraviolet radiations i.e. the sunlight. Due to exposure to the atmosphere, its ability to withstand load is reduced.
Gilder in paper emphasized on the separation of water-oil emulsion. It comprises of minute droplets. The droplets which are very minute having 100 μmare very difficult to separate. Secondary the process of coalescence depends on the properties of the droplets. It depends upon the size and performance of the droplets. This affects the surface area of the emulsion. These fibers are mixed with micro glass fibers to modify the glass fiber filter media. The cellar structure of the polystyrene proves to be highly resistant to the flow of heat. ASTM C578 has excellent thermal properties. This type of polystyrene is employed for thermal insulation.
NO PLAGIARISM POLICY - ORDER NEW LITERATURE REVIEW ON POLYSTYRENE ASSIGNMENT & GET WELL WRITTEN SOLUTIONS DOCUMENTS WITH FREE TURNTIN REPORT!
The combination of nano-composites along with polystyrene produced polypropylene-nitrate Polystyrene is employed in pharmaceuticals in a productive and effective manner. The process of formation of these polymers is not that much difficult. A major portion of the need for thermoplastic is fulfilled by polystyrene. They account for around seven to eight percent of the needs. These materials can be transported by maintaining them in thermal control conditions. The process of formation of these polymers is not that much difficult. The most probable application of these polymers is thin layered sheets, bath sponges and the thin layer of sulfonate. A major portion of the need for thermoplastic is fulfilled by polystyrene. They account for around seven to eight percent of the needs. Under controlled conditions, these properties were tested with the help of scattered diffraction and the high-intensity monochromatic laser microscope was employed to study the combustion residues.
Polystyrene does not get easily dissolved with acids or bases.He stressed upon the drawbacks of employing polystyrene, It can break easily on continuous exposure to ultraviolet radiations i.e. the sunlight. Due to exposure to the atmosphere, its ability to withstand load is reduced. It is used in camper vans where the composition of the door accounts for around 65% of polystyrene while the remaining percent accounts for aluminum. Several investigations have been carried based on these combinations and enhancements in properties have been seen in most cases. The properties of polystyrene were clearly studied before employing this design to the campervans. The manufacturing of polystyrene products is quite easy.
According to Taya.K polystyrene has found its application in the field of construction. ASTM C578 has excellent thermal properties. This type of polystyrene is employed for thermal insulation. These silicates can be employed for thermal insulation. It is also a suitable material that can be employed in the packaging industry. Polystyrene is employed in pharmaceuticals in a productive and effective manner. These materials can be transported by maintaining them in thermal control conditions. The cellar structure of the polystyrene proves to be highly resistant to the flow of heat. The polystyrene's thermal conductivity varies with temperature.
Entine in his study stated the properties of polystyrene for campervans. They are capable of withstanding loads that arise during the opening and closing of doors. These doors are provided by hinges. Zamora in her article stated the importance of weight reduction in vehicles. There have been advancements in technology in day to day basis. Weight reduction plays a major role in deciding the orientation of the vehicle.
The composition of the door accounts for around 32%of polystyrenes while the remaining percent accounts for aluminum. Several materials have been employed to reduce weight in the past. Polystyrene is one of them. About six to eight percent of the fuel efficiency can be enhanced by reducing the weight of the vehicle. Several investigations have been carried based on these combinations and enhancements in properties have been seen in most cases. The properties of polystyrene were clearly studied before employing this design to the campervans. The manufacturing of polystyrene products is quite easy. After analyzing the conditions, these properties were tested with the help of scattered diffraction and the high-intensity monochromatic laser microscope was employed to study the combustion residues. The report gives a brief description of the use of polystyrene to produce a polymer which has reduced flammability characteristics.
ENDLESS SUPPORT IN LITERATURE REVIEW ON POLYSTYRENE ASSIGNMENTS WRITING SERVICES - YOU GET REVISED OR MODIFIED WORK TILL YOU ARE SATISFIED WITH OUR LITERATURE REVIEW ON POLYSTYRENE ASSIGNMENT HELP SERVICES!
Get the best Swinburne University of Technology, Australia Assignment Help Services for related units and courses such as:-
- ADM80010 Advanced CAD/CAM Assignment Help
- ADM80011 Robotics in Manufacturing Assignment Help
- ADM80012 Technology Management Assignment Help
- ADM80013 Advanced Manufacturing Processes Assignment Help
- ADM80014 Intelligent Inspection Systems Assignment Help
- ADM80015 Computer Modelling, Analysis and Visualisation Assignment Help
- ADM80017 Masters Thesis Assignment Help